Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The addition of a crystal of I(2) to NaBr turns the solution violet.
Text Solution
|
- The addition of a crystal of I(2) to NaBr turns the solution violet.
Text Solution
|
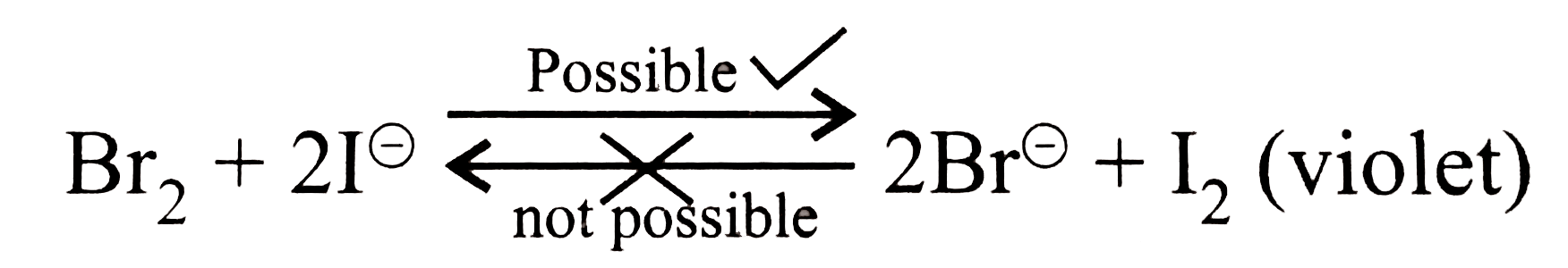
- The addition of Br(2) to NaI turns the solution violet.
Text Solution
|
- The colour of I(2) is violet because it
Text Solution
|
- On addition of ozone gas to KI solution, violet vapours are obtained. ...
Text Solution
|
- Which of the following solutions will turn violet when a drop of lime ...
Text Solution
|
- Aqueous solution of (P) and (Q) are violet. (P) turns green on oxidati...
Text Solution
|
- Which of the following solutions will turn violet when a drop of lime ...
Text Solution
|
- Which of the following solutions will turn violet when a drop of li...
Text Solution
|