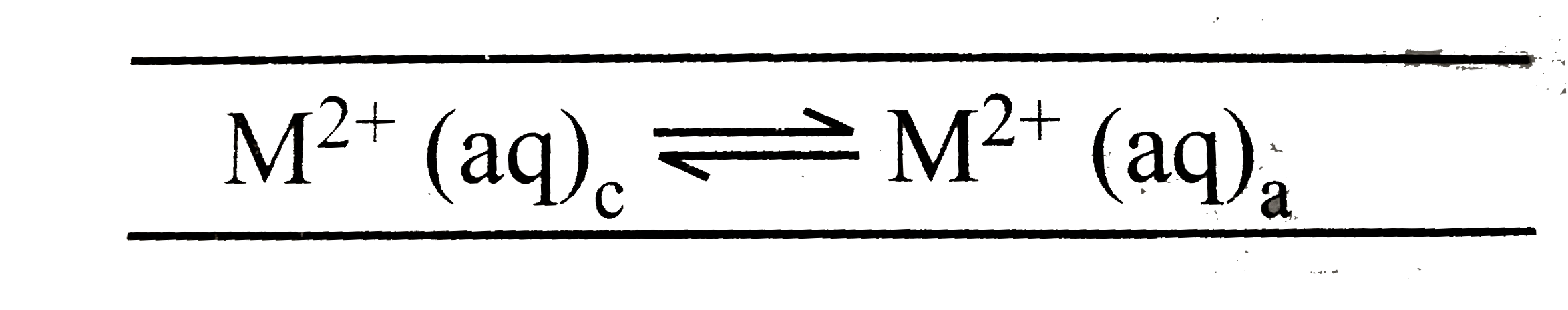
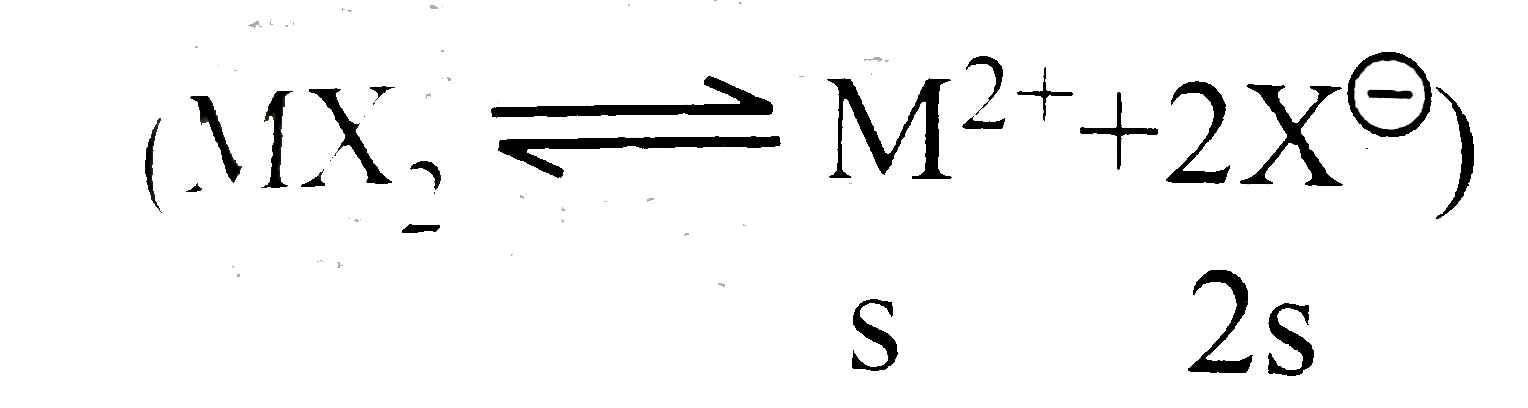A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(2+)...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(2+)...
Text Solution
|
- The electrochemical cell shown below is concentration cell. M|M^(2+) (...
Text Solution
|
- The electrochemical cell shown below is concentration cell. M|M^(2+)...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(...
Text Solution
|