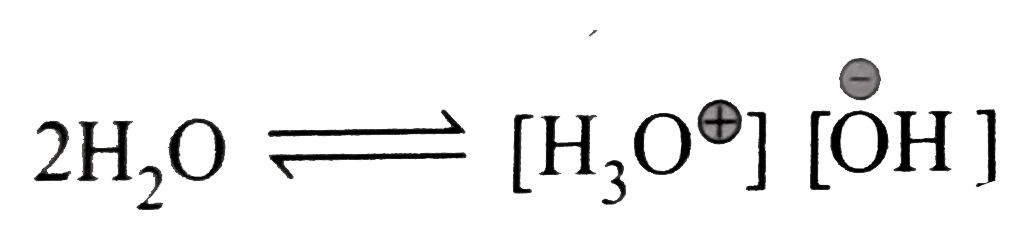
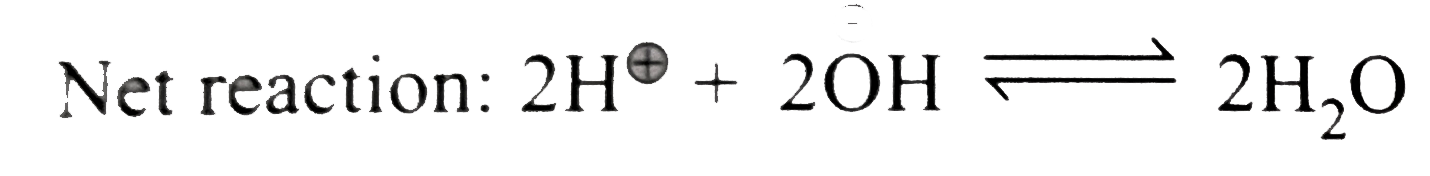Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2...
Text Solution
|
- Use standard enthalpies of formation to calculate the value of Delta(r...
Text Solution
|
- The standard potential of a cell using the reaction +3HgO(s)+2(overse...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2e^...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2e^...
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-H-underset(.^(...
Text Solution
|
- Calculate Delta H ^(@) for the reaction CH(2)= CH(2) + 3O(2) rarr 2CO...
Text Solution
|
- For the reaction, 2H(2)+O(2) to 2H(2)O,DeltaH=571. bond energy of H-H=...
Text Solution
|
- Name the electrode at which the following reduction reaction occurs. W...
Text Solution
|