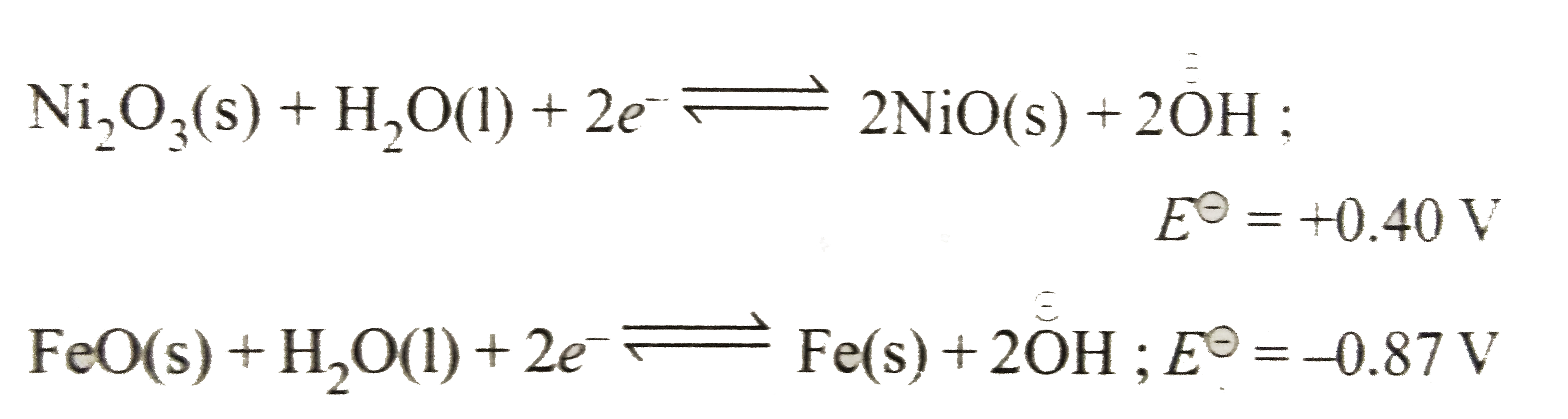Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- The Edison storage cell is represented as Fe(s)|FeO(s)|KOH (aq)|Ni(2...
Text Solution
|
- The Edison storage cell is represented as, Fe((s))|FeO(S)||KOH((aq.))|...
Text Solution
|
- एडीसन संचायक सेल (Edison storage cell) को निम्न प्रकार प्रदर्शित किया ...
Text Solution
|
- Construct at cell consisting of Ni((ag))^(2+)| Ni((s)) half cell an...
Text Solution
|
- The Edision storage cell is represented as Fe(s)|FeO(s)|KOH(aq)|Ni(2)O...
Text Solution
|
- At 25^(@)C equilibrium constant for the reaction Ni((s))+2Ag((aq))^(+)...
Text Solution
|
- The half - cell reactions of a galvanic cell are : Ni(2)O(3)(s)+H(2)...
Text Solution
|