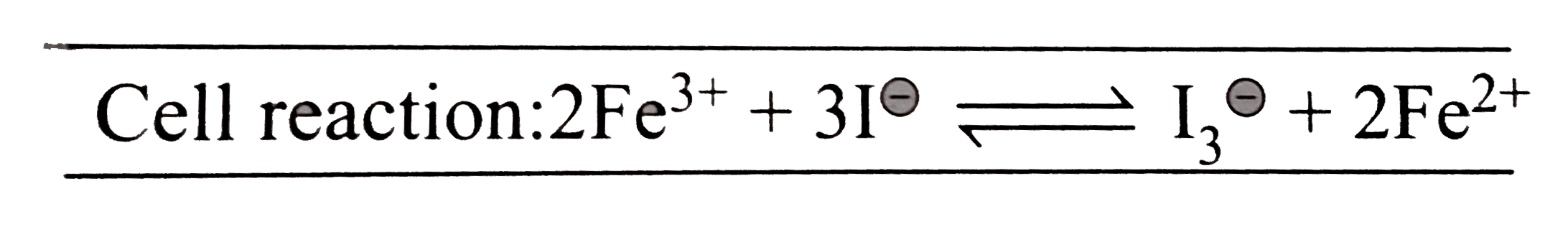Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the equilibrium constant for the reaction : 2Fe^(3+)+3I^(Θ...
Text Solution
|
- Calculate the equilibrium constant for the reaction : 2Fe^(3+)+3I^(Θ)h...
Text Solution
|
- Calculate the euilibrium constant for the reaction, 2Fe^(3+) + 3I^(-) ...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+)+3I^(-)hA...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+) + 3I^(...
Text Solution
|
- The equilibrium constant of the following redox rection at 298 K is 1 ...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+)+3^(-)hAr...
Text Solution
|
- निम्नलिखित अभिक्रिया का साम्य स्थिरांक ज्ञात करो | 2Fe^(3+)+3I^(0)hA...
Text Solution
|
- Calculate the equilibrium constant for the reaction : 2Fe^(3+)+3I^(-)h...
Text Solution
|