Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
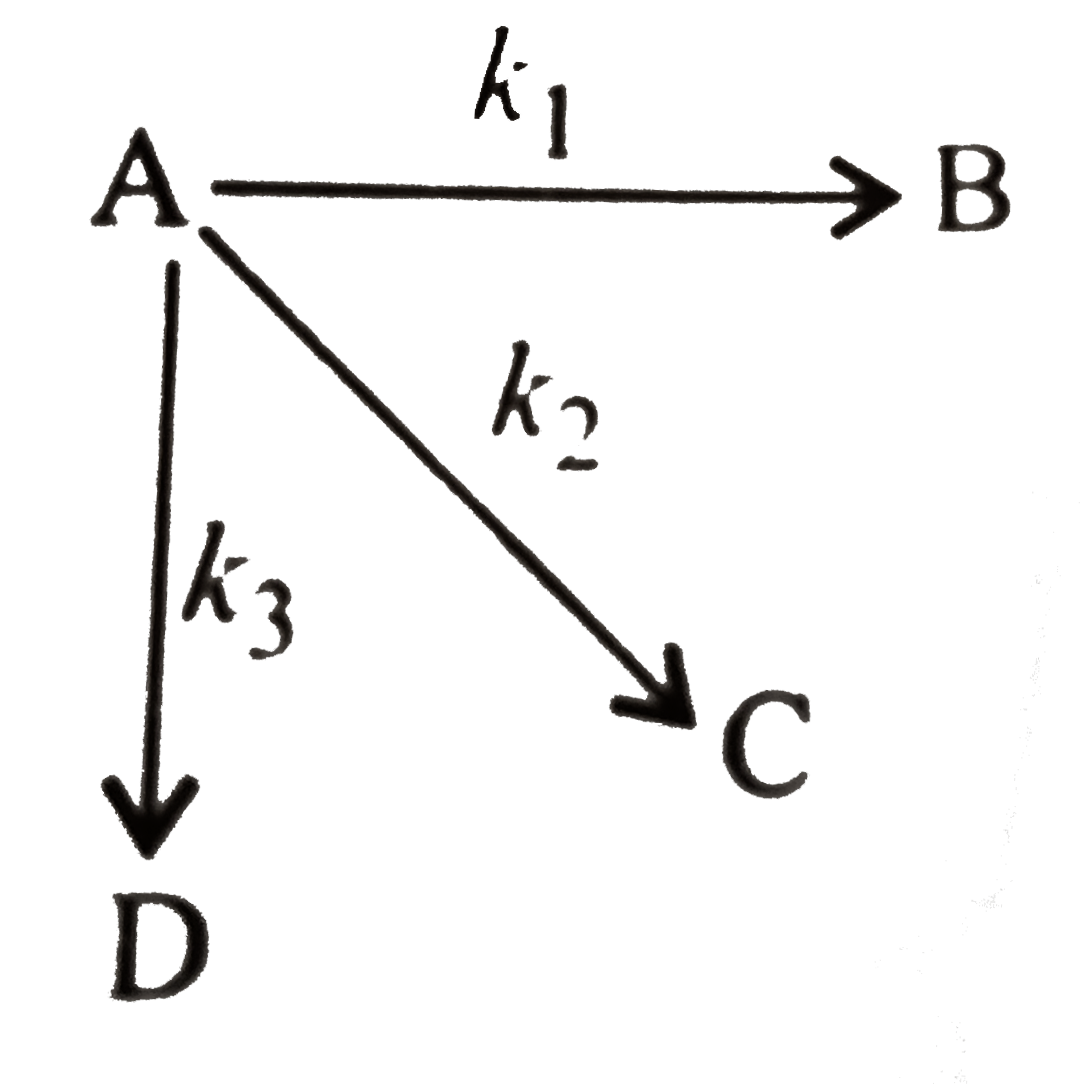
- Mechanism of the reaction is: What is (-d[A])/(dt) ?
Text Solution
|
- Mechanism of the reaction is: A overset(k(1))rarrB, 2Aoverset(k(2))ra...
Text Solution
|
- Mechanism of the reaction is: What is (-d[A])/(dt) ?
Text Solution
|
- Mechanism of the reaction is: A(2) overset(k(2))hArr 2A A + B ove...
Text Solution
|
- Mechanism of the reaction is: What is (a) (-d[A])/(dt), (b) (d[A(...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- N2+3H2hArr2NH3 उपरोक्त अभिक्रिया की दर , -(d[H2])/(dt) तथा (d[NH3])...
Text Solution
|