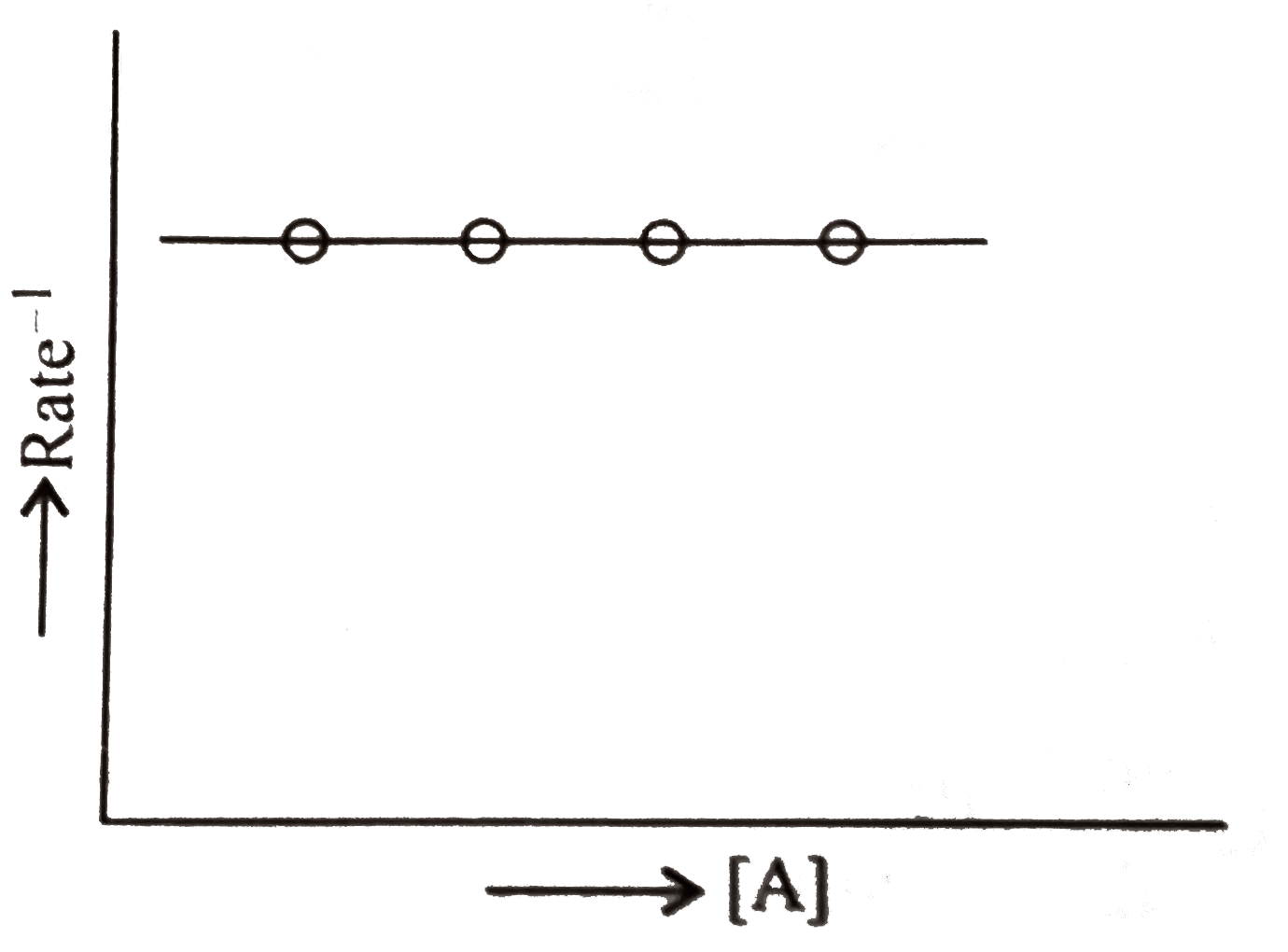
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 2A + 3B rarrProduct a. What is the molecularity of the reaction? b...
Text Solution
|
- 2A + 3B rarr Product a. What is the molecularity of the reaction? ...
Text Solution
|
- For the chemical reaction A + B + C rarr E , The rate of reaction is d...
Text Solution
|
- For reaction A + B rarr products, the rate of the reaction was doubled...
Text Solution
|
- A + 2B to उत्पाद , का अभिक्रिया वेग समीकरण - (d[A])/(dt) = k [A] [B]^...
Text Solution
|
- अभिक्रिया 2A + B rarr उत्पाद, में दोनों अभिकारकों का प्राम्भिक सांद्रण...
Text Solution
|
- For a reaction A+B rarr products, the rate of reaction was doubled whe...
Text Solution
|
- For the reaction: A + B to product (dx)/(dt) = k[A]^(a)[B]^(b) if (d...
Text Solution
|
- Consider the reaction nA+nB to Products, when concentration of both r...
Text Solution
|