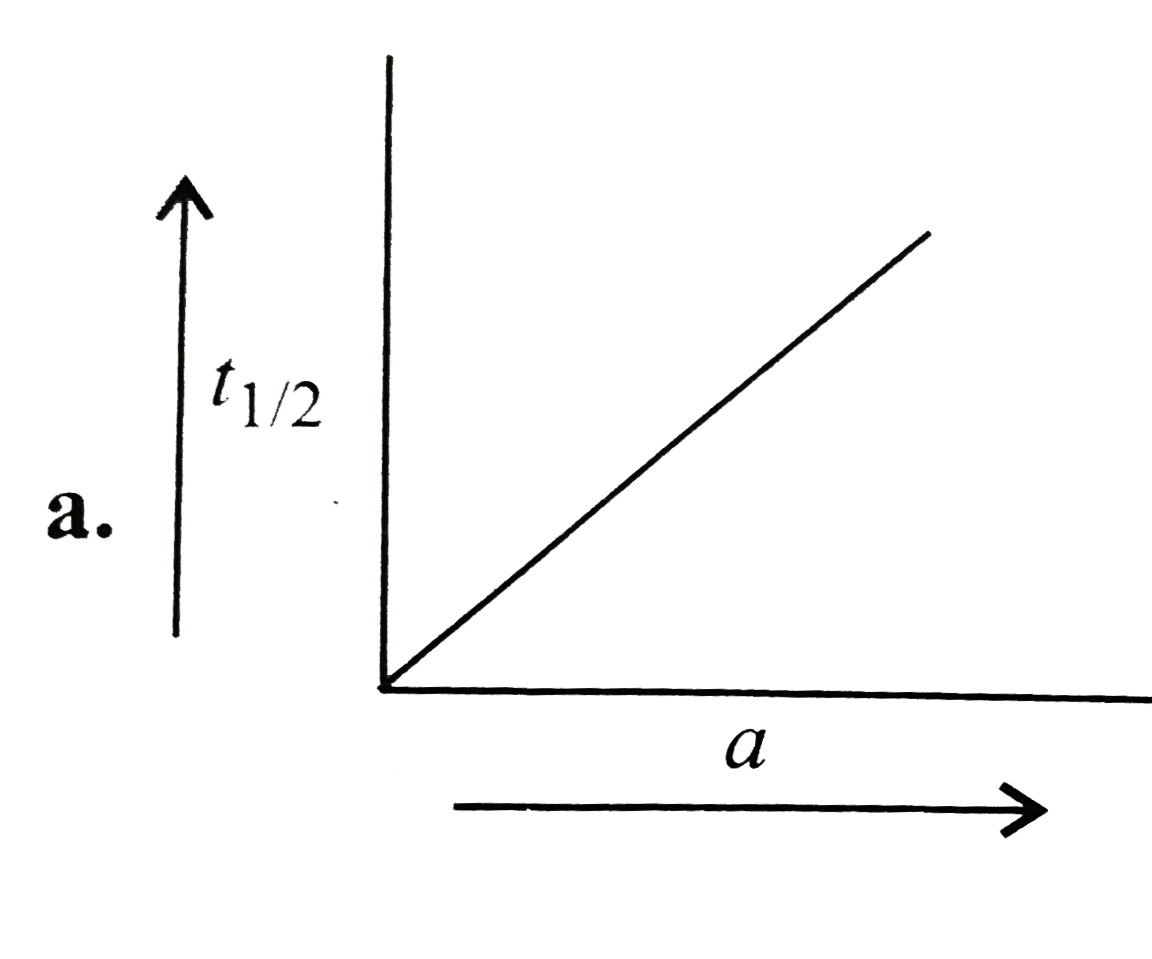
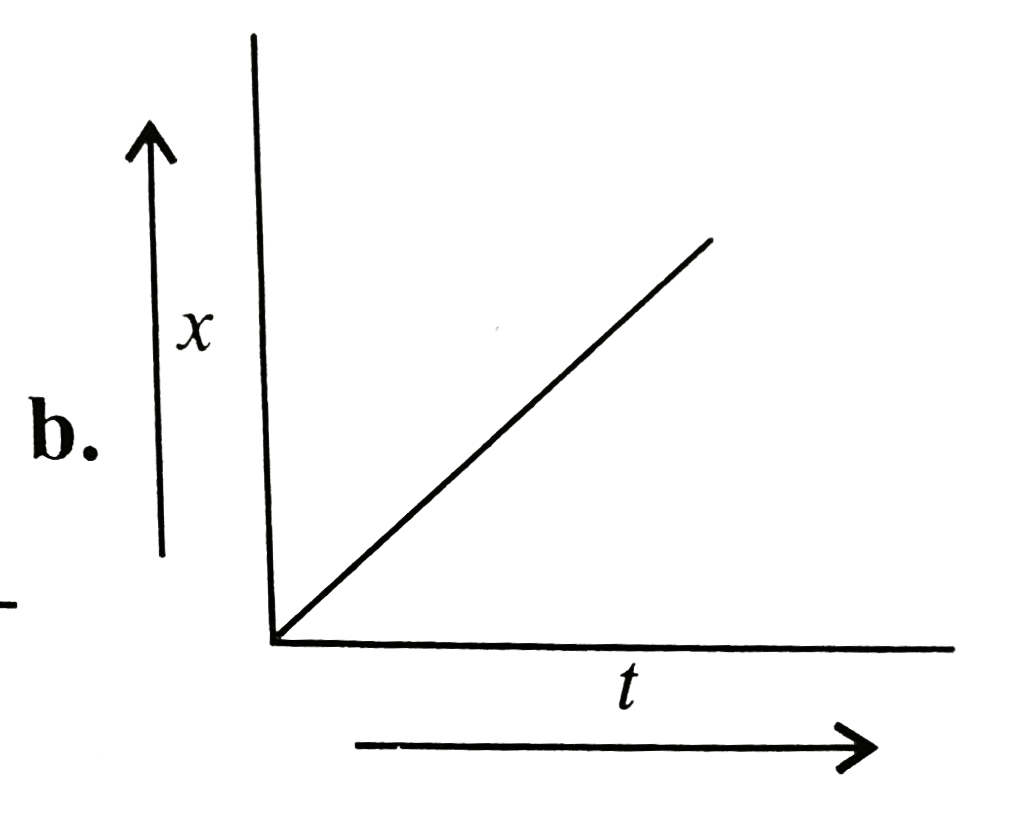
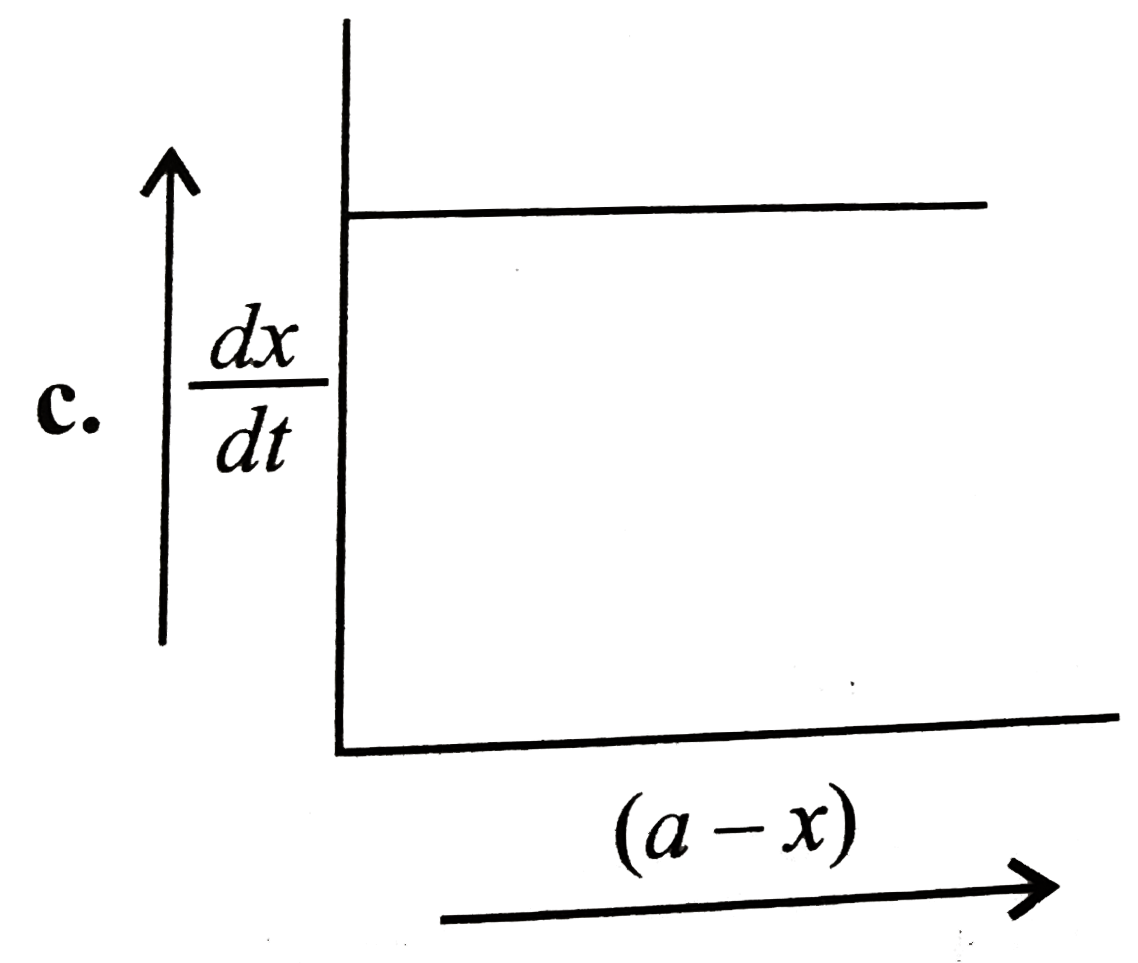
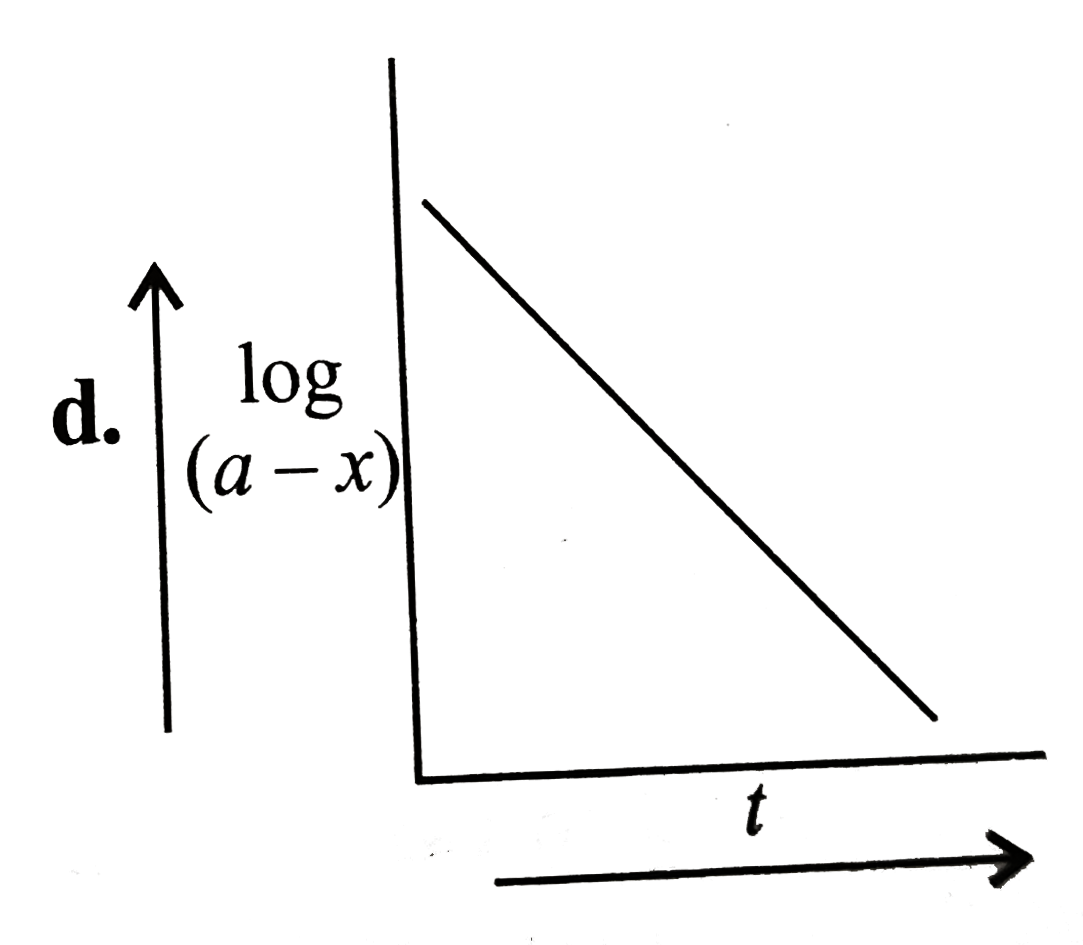
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following graphs is not for zero order reaction?
Text Solution
|
- Which of the following graphs is not for zero order reaction?
Text Solution
|
- Which graph represents zero-order reaction [A(g) rarr B (g)] ?
Text Solution
|
- Which of the following graphs is the characteristic of a zero order re...
Text Solution
|
- Which of the following graph is correct w.r.t half life for a zero ord...
Text Solution
|
- Which of the following graphs is correct for a zero order of reaction?
Text Solution
|
- Which of the following graphs is correct for a zero order reaction ?
Text Solution
|
- Which of the following graphs is correct for a zero order reaction -
Text Solution
|
- Which of the following graph is correct for zero order reaction?
Text Solution
|