Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 2-SECTION B
- Deduce a Charles, law b. Graham's law of diffusion from kinetic gas eq...
Text Solution
|
- Balance the following redox reaction in basic medium by ion-electron m...
Text Solution
|
- What is a conjugate acid-base pair ? Write the conjugate acid and conj...
Text Solution
|
- What is a conjugate acid-base pair ? Write the conjugate acid and conj...
Text Solution
|
- Explain the following with suitable examples : Electron deficient hy...
Text Solution
|
- Explain the following with suitable examples : Ionic hydrides
Text Solution
|
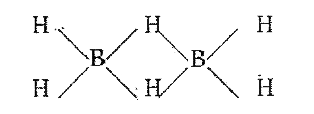
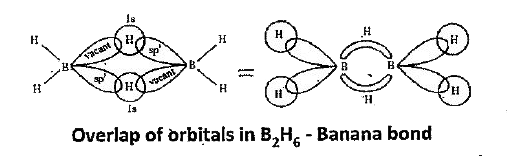
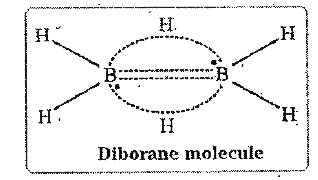
- Explain the structure of diborane.
Text Solution
|
- What do yor understand by Allotropy
Text Solution
|
- What do you understand by Inert pair effect
Text Solution
|
- Describe any two methods of preparation of ethane with equations, give...
Text Solution
|
- Write the reactions of Ethylene with the following : (a) Ozone " ...
Text Solution
|
- Write the reactions of Ethylene with the following : (a) Ozone " ...
Text Solution
|