Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 2-SECTION C
- What are the postulates of Bohr's model of hydrogen atom?
Text Solution
|
- State Hund's rule and Aufbau principle.
Text Solution
|
- Explain how the elements are classified into S. p .d and f- bl...
Text Solution
|
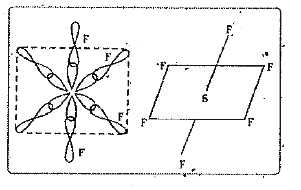
- Explain the hybridisation involved is SF(6).
Text Solution
|
- State Fajan's rules, and give suitable examples.
Text Solution
|