Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 3-Section-B
- Define (a) RMS (b) average and (c) most probable speeds of gas molecul...
Text Solution
|
- Balance the following equation in acid medium by Ion-electron method :...
Text Solution
|
- Calculate the pH of the following basic solutions a. [OH^(-)]=0.05M ...
Text Solution
|
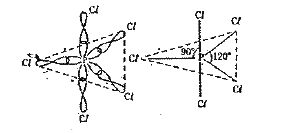
- Explain the hybridization involved in PCl(5) molecule.
Text Solution
|
- Write two oxidizing and two reducing properties of H(2)O(2).
Text Solution
|
- Explain the following : a) Graphite is a good conductor. b) Diamon...
Text Solution
|
- Explain the factors favourable for the formation of cation in lonic bo...
Text Solution
|
- Write any two methods of preparation of diborane. How does it react wi...
Text Solution
|