Hybridisation : " Intermixing of atomic orbitals of almost equal energies of an atom and their redistribution into a equal number of identical orbitals is called, " hybridisation ..
Examples : Let us see the three types hybridisations - `sp^(3),sp^(2)` and sp .
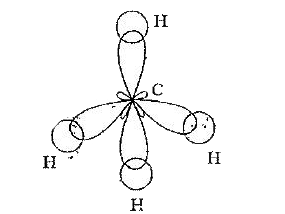
1) `sp^(3)` Hybridisation : The phenomenon of intermixing of one . s . orbital and three . p . orbitals forming four `sp^(3)` hybrid orbitals is called `sp^(3)` hybridisation.
Each of `sp^(3)` hybrid orbitals possess `(1)/(4)` of s - character and `(3)/(4)` of p character. The bond angle in - between any two `sp^(3)` hybrid orbitals is `109^(@)28` . The shape of the molecule in which the central atom undergoes `sp^(3)` hybridisation is tetrahedral.

Example : Formation of Methane `(CH_(4))` molecule :
In the formation of methane molecule the central carbon atom undergoes `sp^(3)` hybridisation in its excited state. As a result of which four `sp^(3)` hybrid orbitals will from on it. All the four `sp^(3)` hybrid orbitals possess bond pair of electrons. Now, the four `sp^(3)` hybrid orbitals overlap head - head with . 1s . orbitals of four . H . atoms forming `CH_(4)` molecule. The shape of the molecule is tetrahedral with a bond angle of `109^(@)28` .
`C(Z)=6 1s^(2)2s^(2)2p^(2)`
Ground state `1s^(2)2s^(2)2p^(1)2p^(1)`
Excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(1))/(sp^(3))`
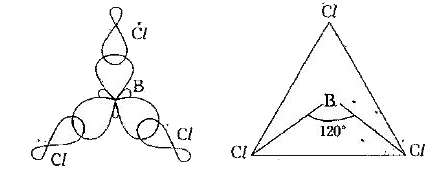
2) `sp^(2)` Hybridisation : The phenomenon of intermixing of one . s . orbital and two . p . orbitals forming three `sp^(2)` hybrid orbitals is called `sp^(2)` hybridisation.
Each of the `sp^(2)` hybrid orbitals possess `(1)/(3)` s - character and `(2)/(3)` p - character. The bond angle in between any two `sp^(2)` hybrid orbitals is `120^(@)` . The shape of the molecule in which the central atom undergoes `sp^(2)` hybridisation is plane triangular.

Example : Formation of Boron trichloride `(BCl_(3))` molecule :
In the formation of `BCl_(3)` molecule the central boron atom undergoes `sp^(2)` hybridisation in its excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(0))`
As a result of which three `sp^(2)` hybrid orbitals will from on it. All the three hybrid orbitals possess an electron each. Now, the three `sp^(2)` hybrid orbitals overlap head - head with half - filled `3p_(z)` orbitals of three chlorine atoms forming `BCl_(3)` molecule. The shape of the molecule is plane triangular with a bond angle of `120^(@)` .
3) sp Hybridisation : The phenomenon of intermixing of one . s . orbital and one . p . orbital of an atom forming two . sp . hybrid orbitals is called sp hybridisation.
Each of sp hybrid orbitals possess `(1)/(2)` s - character and `(1)/(2)` p - character. The bond angle in - between the two hybrid orbitals is `180^(@)` . The shape of the molecule in which the central atom undergoes sp hybridisation is linear.

Example : Formation of Beryllium chloride `(BeCl_(2))` .
In the formation of `BeCl_(2)` molecule the central . Be . atom undergoes sp hybridisation in its excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(0)2p_(z)^(0))`
As a result of which two sp hybrid orbitals wii from on it. The two hybrid orbitals contain an electron, each. Now, the two sp hybrid orbital overlap head - head with half - filled `3p_(z)` orbitals of two chlorine atoms forming `BeCl_(2)` molecule . The shape of the molecule is linear with a bond angle of `180^(@)` .






