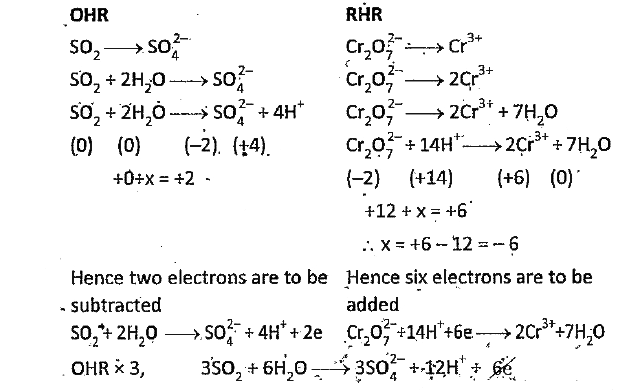
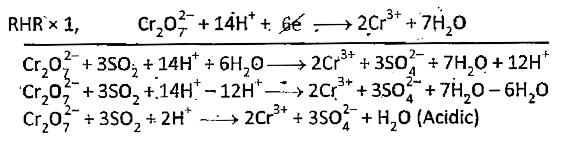
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 4-Section-B
- Explain different types of hydrogen bonds with examples.
Text Solution
|
- What is Hybridization ? Explain the structure of CH4 on the basis of H...
Text Solution
|
- 360 cm^3 of CH4 gas diffused through a porous membrane in 15 minutes....
Text Solution
|
- Balance the following Relox reaction by ion-electron method an acidie ...
Text Solution
|
- State and explain the Hess's law of constant heat summation.
Text Solution
|
- Discuss the application of LE Chatellier's principle for the industria...
Text Solution
|
- Explain the terms hard water and soft water. Write a note on the (ii...
Text Solution
|
- Explain Borax bead test with suitable example.
Text Solution
|