Preparation of Benzene :
1) Laboratory Method : Sodium benzoate on distillation with soda lime gives benzene .
`underset("Sod. benzoate")(C_(6)H_(5)COONa+NaOH)underset(Delta)overset(CaO)(to)underset("Benzene")( C_(6)H_(6)+Na_(2)CO_(3))`
A mixture of NaOH and CaO is called soda lime .
2) Polymerisation of acetylene : When acetylene gas is passed through red hot copper tube, it undergoes polymerisation forming benzene .
`underset("Acetylene")(3C_(2)H_(2))overset("Red hot Cu")underset(600^(@)C)(to) underset("Benzene")(C_(6)H_(6))`
Halogenation and Alkylation reactions of Benzene :
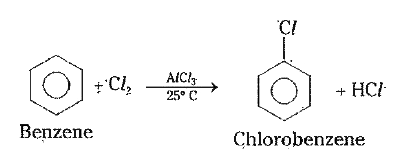
1) Halogenation : In presence of anhydrous `AlCl_(3)` benzene reacts with `Cl_(2)` and gives chloro- benzene .
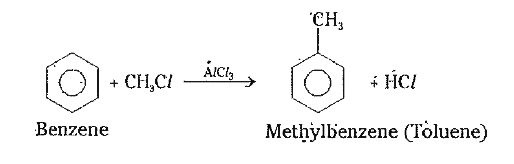
2) Friedel - Craft.s alkylation : In presence of `AlCl_(3)` benzene reacts with alkyl halides and gives alkyl benzene . This is known as Friedel Craft.s alkylation .