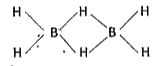1. Boron trifluoride on reduction with LiH at 450 K gives Diborane.
`2BF_(3) + 6 LiH rarr B_(2)H_(6) + 6 LiF`
2. On passing silent electric discharge through a mixture of boron trichloride and hydrogen, diborane is formed.
`2BCl_(3) + 6H_(2) rarr underset("Diborane")(B_(2)H_(6)) + 6HCl`
Molecules in which the central atom gets less than octet configuration are called electron - deficient molecules.
Ex : (1) Diborane `B_(2)H_(6)` (2) Boron trifluoride `BF_(3)`
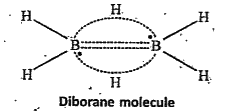
Structure of diborane :
Electron diffraction studies have shown that diborane contains two coplanar `BH_(2)` groups. Its structure can be represented as
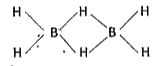
The four H atoms present in the `BH_(2)` groups are known as terminal hydrogen atoms. The remaining two H atoms are called Bridge hydrogens. The two bridge hydrogens lie in a plane perpendicular to the plane of the two `BH_(2)` groups. Out of the two bridge hydrogens, one H atom lie above the plane and the other H atom lies below the plane.
Orbital structure of Diborane : In Diborane each boron atom undergoes `sp^(3)` hybridisation resulting in four equivalent `sp^(3)` hybrid orbitals. Three of these orbitals have one electron each and the fourth hybrid orbital is vacant. In the formation of B - H - B bridge, `sp^(3)` hybrid orbital with one electron from one boron atom, 1s orbital of one bridge hydrogen and the vacant `sp^(3)` hybrid orbital of the second boron atom overlap as shown below.
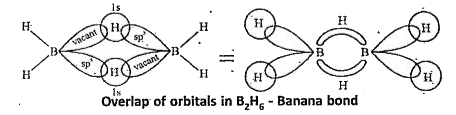
The two B-H-B bridges present in Diborane are abnormal bonds. They are considered as three centered two electron bonds. This type of bond is called banana bond or tau bond. In this type of bond a pair of electrons holds three atoms. In Diborane there are two such bridges. This Diborane structure can also be represented as shown below.