The concept of H-bond is due to Huggins, Latimer and Rodebush. Def : "The electrostatic force of attraction between a partially charged hydrogen atom of a molecule and a highly electronegative atom of the same molecule or different molecule is known as H-bond."
Explanation : 1. The molecule having H-bonds should have a highly electronegative atom like F, O or N directly linked to H-atom by a covalent bond.
2. H-bond is stronger than van der waal.s forces but weaker than ionic and covalent bonds.
van der Waal.s forces `lt` H-bond `lt` Covalent bond `lt` Ionic bond
Energy : 2 - 10 kJ `mol^(-1)` 2 - 40 kJ `mol^(-1)` 200 - 400 kJ `mol^(-1)`
3. The strength of H-bond increases with the EN of the atom attached covalently to H atom.
Types of H-bonds : 2 types. (1) Intramolecular H-bonding and (2) Inter-molecular H-bonding
1. Intramolecular H-bonding : The H-bonding formed between H atom of a molecule and a highly elecronegative atom of the same molecule is called intramolecular hydrogen bond.
Ex :
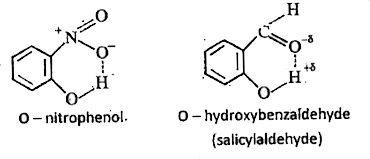
2. Inter-molecular H-bonding : The H-bonding formed in between H-atom of one molecule and a highly electronegative atom of another molecule of same substance or different substances substances is called intermolecular hydrogen bond.
Ex :
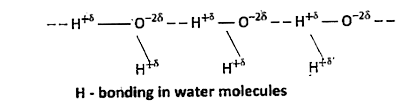
Consequences of H-bonding :
1. Highly viscous nature of `H_(2) SO_(4), HNO_(3), H_(3)PO_(4)` etc, is due to H-bonding.
2. The high B.P. of water is due to H-bonds in it.
3. `H_(2)O` is a liquid but `H_(2)S` is a gas, since H-bond is present in `H_(2)O` only.
4. The solubility of alcohols, carbohydrates etc. in water is due to formation of H-bonds with water molecules.
5. B.P. of ethyl alcohol is more than that of dimethyl ether of equal molecular weight because there is H-bonding in ethyl alcohol but not in ether.

