How are the elements divided into s, p, d and f – blocks in the Modern periodic table ?
How are the elements divided into s, p, d and f – blocks in the Modern periodic table ?
Text Solution
Verified by Experts
Depending upon the entering of differentiating electrn into the atomic orbitals, the elements of long form of periodic table are divided into four blocks. They are s, p, d and f-blocks.
s-block : The elements, in which the differentiating electron enters the s- sub-level are called s-block elements.
In this block, there are two groups. They are IA and IIA.
The valence electron configuration of elements of IA group is `ns^(1)` and that of IIA is `ns^(2)`
p-Block : The elements in which the differentiating electron enters the p- sub-level are called p-block elements. In this block, there are six groups. They are IIIA, IVA, VA, VIA, VIIA and .0.. The valence electron configuration of the elements of these groups varies from `ns^(2) np^(1)` to `ns^(2) np^(6)`
d-Block : The elements in which the differentiating electron enters into d- sub-level are called d-block elements. In this block, there are then groups. They are IIIB, IVB, VB, VIB, VIIB, VIII, IB, IIB. The general outer configuration of d-block elements is `(n - 1)d^(1 - 10) ns^(1-2)`. These elements are also known as Transition elements.
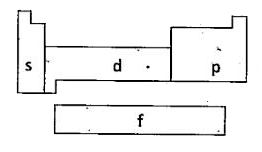
f-Block : The elements in which the differentiating electron enters into f-orbital are called f-block elements. In this block, there are 14 groups. They have no designation. Their general outer configuration is `(n - 2) f^(1-14) (n - 1)d^(0 - 1) ns^(2)`. The f-block elements are arranged separately at the bottom in two rows. The elements of first row are called lanthanides and in these elements the valence electron enters into 4f orbital. The elements of second row are called actinides and in these elements the valence electron enters into 5f orbital. All the f-block elements are also known as Inner Transltion elements.
Advantages of this type of classification :
1. There is a good correlation between the properties of the element and its position in the periodic table.
2. A gradual gradation in the physical and chemical properties of elements is clearly observed.
3. As we go grom elements of s-block to elements of p-block, a gradual decrease in the metallic nature and a gradual increase in the non-metallic nature is observed.
4. Special position for radioactive elements is given.
Anomalies : There are two anomalies
(i) Helium : He has `1s^(2)` configuration. So it belongs to .s. block. But it is included in inert elements of p-block. But this is justified for two reasons.
(a) It has completely filled valence shell `(1s^(2))` like the inert gases.
(b) Because of such configuration, it exhibits characteristic properties of the inert gases.
(ii) Hydrogen : Its configuration is `1s^(1)`. So it can be placed in `1^(st)` group along with alkali metals. But like the halogens `(ns^(2) np^(5))`, it is short of 1 electron for the inert gas structure.
s-block : The elements, in which the differentiating electron enters the s- sub-level are called s-block elements.
In this block, there are two groups. They are IA and IIA.
The valence electron configuration of elements of IA group is `ns^(1)` and that of IIA is `ns^(2)`
p-Block : The elements in which the differentiating electron enters the p- sub-level are called p-block elements. In this block, there are six groups. They are IIIA, IVA, VA, VIA, VIIA and .0.. The valence electron configuration of the elements of these groups varies from `ns^(2) np^(1)` to `ns^(2) np^(6)`
d-Block : The elements in which the differentiating electron enters into d- sub-level are called d-block elements. In this block, there are then groups. They are IIIB, IVB, VB, VIB, VIIB, VIII, IB, IIB. The general outer configuration of d-block elements is `(n - 1)d^(1 - 10) ns^(1-2)`. These elements are also known as Transition elements.

f-Block : The elements in which the differentiating electron enters into f-orbital are called f-block elements. In this block, there are 14 groups. They have no designation. Their general outer configuration is `(n - 2) f^(1-14) (n - 1)d^(0 - 1) ns^(2)`. The f-block elements are arranged separately at the bottom in two rows. The elements of first row are called lanthanides and in these elements the valence electron enters into 4f orbital. The elements of second row are called actinides and in these elements the valence electron enters into 5f orbital. All the f-block elements are also known as Inner Transltion elements.
Advantages of this type of classification :
1. There is a good correlation between the properties of the element and its position in the periodic table.
2. A gradual gradation in the physical and chemical properties of elements is clearly observed.
3. As we go grom elements of s-block to elements of p-block, a gradual decrease in the metallic nature and a gradual increase in the non-metallic nature is observed.
4. Special position for radioactive elements is given.
Anomalies : There are two anomalies
(i) Helium : He has `1s^(2)` configuration. So it belongs to .s. block. But it is included in inert elements of p-block. But this is justified for two reasons.
(a) It has completely filled valence shell `(1s^(2))` like the inert gases.
(b) Because of such configuration, it exhibits characteristic properties of the inert gases.
(ii) Hydrogen : Its configuration is `1s^(1)`. So it can be placed in `1^(st)` group along with alkali metals. But like the halogens `(ns^(2) np^(5))`, it is short of 1 electron for the inert gas structure.
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
Explain how the elements are classified into s,p,d, and f - block elements in the periodic table and give the advantages of this kind of classification
How are the elements arranged into groups and periods in the modern periodic Table ? Elements in a group posses similar properties , but elements in a period do not show similarites in their properties . Why ?
Explain how the elements are classified into S. p .d and f- block elements in the periodic table and give the advantage of this kind of classification .
How many blocks are there in modern peridic table ?
What is a period in modern periodic table ?
Without knowning the electronic configurations of the above of the atoms of elements Mendeleeff still Could arrange the elements nearly close to the arrangements in the modern periodic table . How can you appreciate this ?
How many periods and groups are there in the modern periodic table ?
Write an essay on the division of elements into s, p, d and f - blocks.