Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 4-SECTION-C
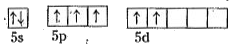
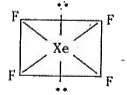
- Explain the structures of XeF(4)
Text Solution
|
- What are galvanic cells ? Explain the woriking of a galvanic cell with...
Text Solution
|
- Write the difference between Order and Molecularity of a reaction.
Text Solution
|
- How is ammonia manufactured by Haber's process ? Explain the reactions...
Text Solution
|
- How is ozone prepared ? How does it react with the following ? PbS
Text Solution
|
- How is chlorine obtained in the laboratory ? How does it react with th...
Text Solution
|
- How does ozone react with Ethylene ?
Text Solution
|
- How does ozone react with the following: NO
Text Solution
|
- Write the equations involved in the following reactions: (i) Reimer ...
Text Solution
|
- Describe the Cannizaro reaction
Text Solution
|
- Explain crossed aldol condensation with suitable examples
Text Solution
|
- Explain the following name reactions : Sandmeyer reaction
Text Solution
|