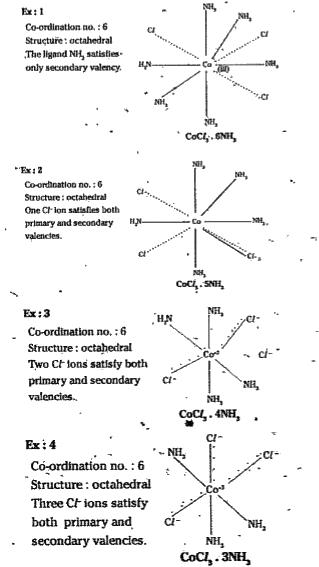
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 4-SECTION-B
- What is the formula of carnallite?
Text Solution
|
- Explain the purification of sulphide ore by Froth Floatation Method.
Text Solution
|
- The vapour pressure of pure benzene at a certain temperature is 0.850 ...
Text Solution
|
- Describe the purification of colloidal solution by the phenomenon of d...
Text Solution
|
- Write equations for the reaction of acetic acid with reagent : NaOH
Text Solution
|
- How is chlorine obtained in the laboratory ? How does it react with th...
Text Solution
|
- Write the equations for.reactions of chlorine with the following : ...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- Explain the applications of Co-ordination compounds in different field...
Text Solution
|
- What are Hormones ? Give one example for each. i) Steroid Hormones ...
Text Solution
|
- What are Hormones ? Give one example for steroid hormones and polypept...
Text Solution
|
- What are Hormones ? Give one example for each. i) Steroid Hormones ...
Text Solution
|
- Explain sp^2 hybridization with an example.
Text Solution
|