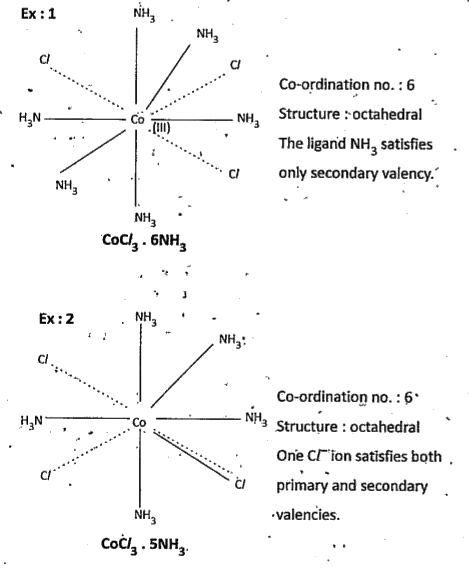
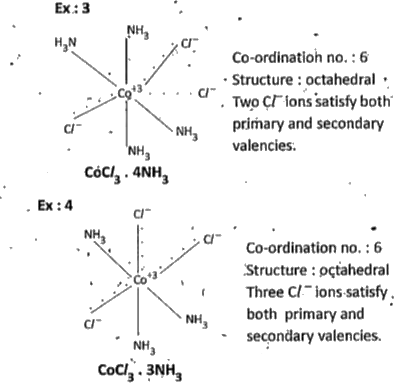
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 6-SECTION - B
- Calculate the mole fraction of H(2)SO(4) in a solution containing 98% ...
Text Solution
|
- Derive Bragg's equation .
Text Solution
|
- What are emulsion ? How are they classified ? Describe the application...
Text Solution
|
- Explain the purification of sulphide ore by Froth Floatation Method.
Text Solution
|
- Give the sources of the following vitamin and name the diseases cause...
Text Solution
|
- Explain ionic bond with suitable example.
Text Solution
|
- How are XeF(2) and XeF(4) prepared ? Give their structures.
Text Solution
|
- Explain the following name reactions : Sandmeyer reaction
Text Solution
|
- Explain the following name reactions : Gatterman reaction
Text Solution
|