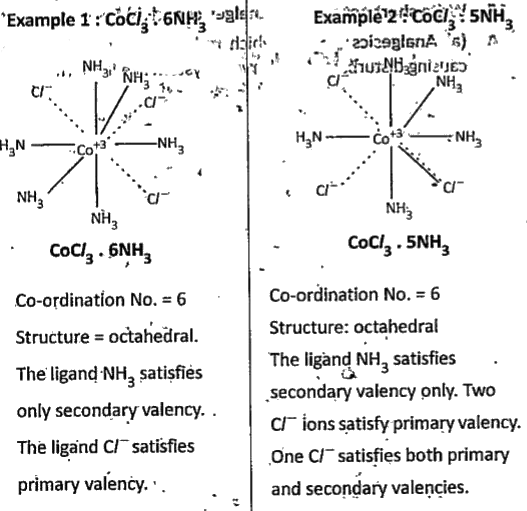
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER -Section - B
- Calculate the vapour pressure of a solution containing 9g of glucose i...
Text Solution
|
- What are the types of systems? Explain give one example each?
Text Solution
|
- Give examples to differentiate roasting and calcination.
Text Solution
|
- Explain the structures of XeF(4)
Text Solution
|
- Explain the structures of XeOF(4)
Text Solution
|
- Explain ionic bond with suitable example.
Text Solution
|
- Give the sources of the following vitamin and name the diseases cause...
Text Solution
|
- Write short notes on Analgesics
Text Solution
|
- Write the defination of binary solution?
Text Solution
|
- Explain the following name reactions : Sandmeyer reaction
Text Solution
|
- (A): Isocyanides are prepared by carbylamine reaction. (R) : Carbyl...
Text Solution
|