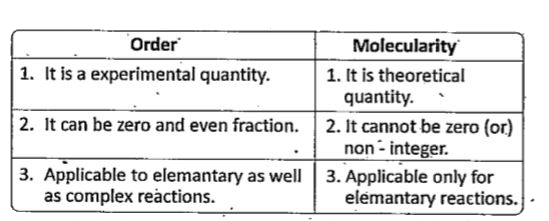
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER -Section - C
- State and explain Kohlrausch's law of indendent migration of ions.
Text Solution
|
- What is Zero Order reaction ?
Text Solution
|
- How is ozone prepared ? How does it react with the following ? KI
Text Solution
|
- Write balanced equations for the folliowing. NaCl is heated with "C...
Text Solution
|
- Write balanced equations for the folliowing. Chlorine is passed int...
Text Solution
|
- Describe the Cannizaro reaction
Text Solution
|
- Describe the following: (i) Acetylation (ii) Cannizzaro reaction ...
Text Solution
|
- Explain the acidic nature of phenols and compare with that of alcohols...
Text Solution
|