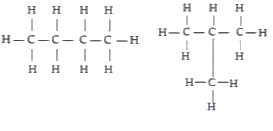
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON COMPOUNDS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT (OBJECTIVE TYPE QUESTIONS Questions) C . Very Short Answer Type Questions |1 VideosCARBON COMPOUNDS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT (OBJECTIVE TYPE QUESTIONS Questions) D. Very Short Answer Type Questions |4 VideosCARBON COMPOUNDS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT (OBJECTIVE TYPE QUESTIONS) A. Multiple Choice Questions|3 VideosCARBON AND ITS COMPOUNDS
OSWAL PUBLICATION|Exercise CREATING BASED QUESTIONS|23 VideosCHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise BOARD CORNER (Short Answer Type Questions) |3 Videos
Similar Questions
Explore conceptually related problems