Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON COMPOUNDS
OSWAL PUBLICATION|Exercise BOARD CORNER (Very Short Answer Type Questions)|5 VideosCARBON COMPOUNDS
OSWAL PUBLICATION|Exercise BOARD CORNER ( Short Answer Type Questions)|23 VideosCARBON COMPOUNDS
OSWAL PUBLICATION|Exercise NCERT EXEMPLAR (Short Answer Questions)|32 VideosCARBON AND ITS COMPOUNDS
OSWAL PUBLICATION|Exercise CREATING BASED QUESTIONS|23 VideosCHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise BOARD CORNER (Short Answer Type Questions) |3 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-CARBON COMPOUNDS -NCERT EXEMPLAR (Long Answer Questions)
- Give the structural differences between saturated and unsaturated hydr...
Text Solution
|
- What is a functional group? Give examples of four different functional...
Text Solution
|
- Name the reaction which is commonly used in the conversion of vegetabl...
Text Solution
|
- Write the formula and draw electron dot structure of carbon tetrachlor...
Text Solution
|
- What is saponification? Write the reaction involved in this process.
Text Solution
|
- Esters are sweet-smelling substances and are used in making perfumes. ...
Text Solution
|
- A compound C (molecular formula, C(2)H(4)O(2)) reacts with Na metal to...
Text Solution
|
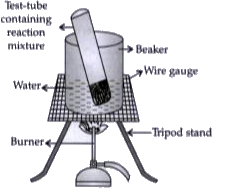
- Look at figure and answer the following questions: What change w...
Text Solution
|
- Look at figure and answer the following questions: Write the rea...
Text Solution
|
- Look at figure and answer the following questions: If ethanol is...
Text Solution
|
- Look at figure and answer the following questions: How can a sol...
Text Solution
|
- How would bring about the following conversions ? Name the process and...
Text Solution
|
- How would bring about the following conversions ? Name the process and...
Text Solution
|
- Draw the possible isomers of the compound with molecular formula C(3)H...
Text Solution
|
- Explain the given reaction with the examples : Hydrogenation reacti...
Text Solution
|
- Explain the following reactions with examples : Oxidation reaction
Text Solution
|
- Explain the given reaction with the examples : Substitution reactio...
Text Solution
|
- Explain the given reaction with the examples : Saponification reacti...
Text Solution
|
- Explain the following reactions with examples : Combustion reaction
Text Solution
|
- An organic compound A on heating with concentrated H(2)SO(4) forms a c...
Text Solution
|