Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-CARBON COMPOUNDS -BOARD CORNER ( Long Answer Type Questions)
- Write the chemical formula and name of the compound which is the activ...
Text Solution
|
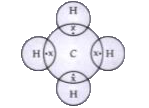
- What is methane? Draw its electron dot structure. Name the type of bon...
Text Solution
|
- Both soap and detergent are some type of salts. What is the difference...
Text Solution
|
- Why are certain compounds called hydrocarbons? Write the general formu...
Text Solution
|
 Covalent bonds
Covalent bonds