Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise NCERT CORNER (INTEXT CORNER)|16 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise NCERT CORNER (EXERCISE QUESTIONS)|19 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise SELF - ASSESSMENT - 1 ( OBJECTIVE TYPE QUESTIONS) |13 VideosOLYMPIAD 2019 - 20
OSWAL PUBLICATION|Exercise CHEMISTRY QUESTIONS (Is Matter Around us Pure)|1 VideosSAMPLE PAPER 1
OSWAL PUBLICATION|Exercise QUESTION BANK|9 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -SELF - ASSESSMENT - 2 ( OBJECTIVE TYPE QUESTIONS)
- The positions of four elements A, B, C and Din the modern periodic tab...
Text Solution
|
- Elements P, Q R and S have atomic numbers 11, 15, 17 and 18 respective...
Text Solution
|
- Arrange the given elements in increasing order of their atomic radii? ...
Text Solution
|
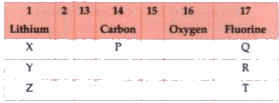
- From the following part of the periodic table, answer the following qu...
Text Solution
|
- From the following part of the periodic table, answer the following qu...
Text Solution
|
- From the following part of the periodic table, answer the following qu...
Text Solution
|
- From the following part of the periodic table, answer the following qu...
Text Solution
|
- Directions : In the following questions, a statement of assertion (A) ...
Text Solution
|
- Directions : In the following questions, a statement of assertion (A) ...
Text Solution
|
- How does valency of an element vary across a period?
Text Solution
|
- Name two elements of first period in modern periodic table.
Text Solution
|
- The atomic numbers of three elements X, Y and Z are 3, 11 and 17, resp...
Text Solution
|
- Two elements X and Y have atomic numbers 12 and 16 respectively. To wh...
Text Solution
|
- The electronic configuration of an element 'X' is 2, 8, 6. To which gr...
Text Solution
|
- An element ''X'' has mass number 35 and number of neutrons is 18. Iden...
Text Solution
|