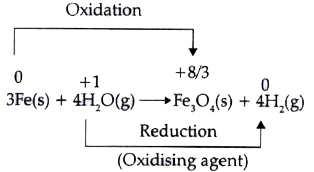
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NTSE 2019 - 20
OSWAL PUBLICATION|Exercise (CHEMISTRY) CHEMICAL REACTION AND EQUATIONS (STAGE 2)|2 VideosNTSE 2019 - 20
OSWAL PUBLICATION|Exercise (CHEMISTRY) ACIDS, BASES AND SALTS (STAGE-1)|5 VideosNTSE 2019 - 20
OSWAL PUBLICATION|Exercise (CHEMISTRY) PERIODIC CLASSIFICATION OF ELEMENTS (STAGE - 1)|5 VideosNEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise PERIODIC CLASSIFICATION OF ELEMENTS (VISUAL CASE - BASED QUESTIONS)|15 VideosOLYMPIAD 2019 - 20
OSWAL PUBLICATION|Exercise CHEMISTRY QUESTIONS (Is Matter Around us Pure)|1 Videos
Similar Questions
Explore conceptually related problems