A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
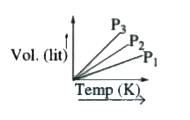
- From the graph the order of pressure of a gas is
Text Solution
|
- In order to increase the volume of a gas by 10% , the pressure of the ...
Text Solution
|
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- In order to increase the volume of a gas by 10% the pressure of the ga...
Text Solution
|
- The given P-U graph shows the variation of internal energy of an ideal...
Text Solution
|
- The following graph is obtained for physisorption of a gas as a functi...
Text Solution
|
- In order to increase the volume of a gas by 10%, the pressure of the g...
Text Solution
|
- In order to increase the volume of a gas by 10%, the pressure of the g...
Text Solution
|
- From the graph the order of pressure of a gas is
Text Solution
|