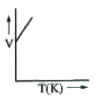
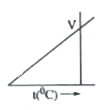
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following shows correct relation between volume and tempe...
Text Solution
|
- At constant temperature the product of pressure and volume of a given ...
Text Solution
|
- Which of the following curve is correct for a given amount of an ideal...
Text Solution
|
- Which of the following shows the correct relationship between the pres...
Text Solution
|
- Which of the following is a correct plot of the volume of fixed amount...
Text Solution
|
- Name the gas law which relates volume and pressure of gas at constant ...
Text Solution
|
- Which of the following shows correct relation between volume and tempe...
Text Solution
|
- Derive the relation between specific heats of a gas at constant pressu...
Text Solution
|
- What is the relation between pressure and temperature of a gas at cons...
Text Solution
|