A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- By taking two J- tubes at constant temperature what is the difference ...
Text Solution
|
- In a constant volume gas thermometer, the pressure of the working gas ...
Text Solution
|
- in previous question, if 15 cm of water and spirit each are further po...
Text Solution
|
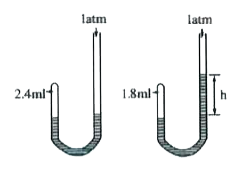
- Given J-tube has 2.4 mL of air at a pressure of 1 atm. On adding mercu...
Text Solution
|
- A U-tube is filled with mercury. When two limbs are maintained at temp...
Text Solution
|
- Some gas is present in J-shaped tube and in this case level of mecury ...
Text Solution
|
- A horizontal tube of 1m is closed at both ends. A mercury column of 20...
Text Solution
|
- In the previous problem, If 15.0 cm of water and spirit each are furth...
Text Solution
|
- In the previous problem, If 15.0 cm of water and spirit each are furth...
Text Solution
|