A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
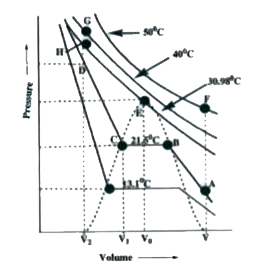
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of CO(2)...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of CO(2)...
Text Solution
|
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|