A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
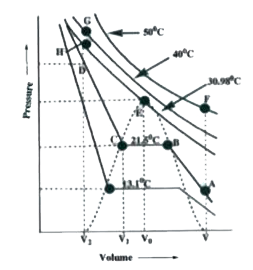
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|
- Which of the following compounds gives carbondioxide with NaHCO(3)?
Text Solution
|
- Atomicity of carbondioxide is .
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Why did we study two leaves in this (Carbondioxide is necessary for ph...
Text Solution
|
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|
- Study the following isotherms of carbondioxide at various temperature ...
Text Solution
|
- Carbondioxide is isostructural with
Text Solution
|