A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
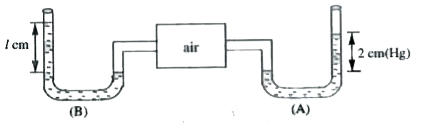
- What is l if the liquid in manometre 'B' has a density 1.36 gm/cc.
Text Solution
|
- A volatile subsatnces A has density of liquid =2 gm/ml at its boiling ...
Text Solution
|
- Molarity of liquid HCl with density equal to 1.17g/cc is
Text Solution
|
- 10 cc of a liquid A were mixed with 10 cc of liquid B. The volume of t...
Text Solution
|
- There are three different liquids ( liquid 1 , liquid 2 and liquid 3) ...
Text Solution
|
- Two misccible liquids of densities 1.2 gm/cc and 1.4 gm/cc are mixed w...
Text Solution
|
- यदि किसी विलयन का घनत्व 1.17 ग्राम/cc है, तो द्रवित HCI की मोलरता क्या...
Text Solution
|
- एक लकड़ी का गुटका पारे (घनत्व 13.6 gm/cc) तथा जल (घनत्व 1gm/cc) दोनों ...
Text Solution
|
- Brine has a density of 1.2 g/cc. 40 cc of it are mixed with 30 cc of w...
Text Solution
|