A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
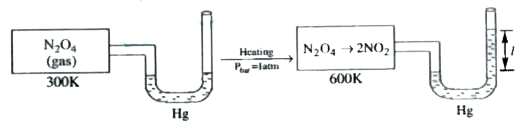
- If the value of l in the above diagram is 114 cm. What is the percenta...
Text Solution
|
- For the reaction N2O4 hArr 2NO2(g) , if percentage dissociation of N2O...
Text Solution
|
- The ratio of the rate of diffusion of a sample of N2O4 partially disso...
Text Solution
|
- N2O4(g) hArr 2NO2(g) . In this reaction, NO2 is 20% of the total volum...
Text Solution
|
- For the reaction , N2O4(g) hArr2NO2(g) , if percentage dissociation of...
Text Solution
|
- Vapour density of the equilibrium mixture of NO(2) and N(2)O(4) is fou...
Text Solution
|
- One mole of N2O4(g) at 300 K I kept in a closed container under one a...
Text Solution
|
- The vapour density of N(2)O(4) at a certain temperature is 30. Calcula...
Text Solution
|
- At 800K, Kp for the reaction: N2O4 hArr 2NO2 is 700 mm. Calculate ...
Text Solution
|