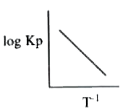
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Variation of K(p) with temperature T is given by for the following equ...
Text Solution
|
- For the reaction, N(2)(g)+3H(2)(g) hArr 2NH(3)(g) , the units of K(p) ...
Text Solution
|
- For the reaction 2NH(3)(g) hArr N(2)(g) +3H(2)(g) the units of K(p) ...
Text Solution
|
- Write the relation between K(p) " and " K(c) for the reaction: N(2)(g)...
Text Solution
|
- Assertion: For the 2NH(3(g))hArr N(2(g))+3H(2(g)), the unit of K(p) wi...
Text Solution
|
- अभिक्रिया N(2)(g)+3H(2)(g)hArr के लिए 400K पर K(p)=41 है। निम्नलिखित म...
Text Solution
|
- अभिक्रिया N(2)(g)+3H(2)(g)hArr 2NH(3)(g) के लिए K(p)=K(c)(RT) ...........
Text Solution
|
- Derive the relation between K(p) and K(c) for the equilibrium reaction...
Text Solution
|
- In the reaction N(2)(g)+3H(2)(g)hArr 2NH(3)(g) , the value of the equl...
Text Solution
|