Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
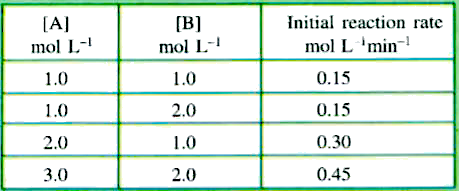
- For a reaction A + 2B to 2C, the kinetic data is given below: ...
Text Solution
|
- अभिक्रिया ,A +2B to 2C से निम्नलिखित तथ्य प्राप्त हुए- इस अभिक...
Text Solution
|
- अभिक्रिया A+2B to 2C के लिए निम्न उपादेय (data) प्राप्त होते है- व...
Text Solution
|
- A+2B to C +D for a reaction from following data correct rat...
Text Solution
|
- Form the data given below for the reaction:A to B to Products the r...
Text Solution
|
- For a reaction A + 2B to 2C , the kinetic data is given below: Determi...
Text Solution
|
- Write the rate law for a first order reaction.
Text Solution
|
- For the chemical reaction A + 2B rarr 2C + D the experimentally determ...
Text Solution
|
- Consider the reaction 2A+2B rarr 2C +D. From the following data, calcu...
Text Solution
|