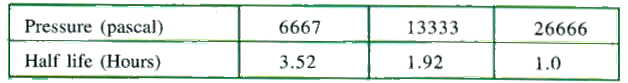Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the catalytic decomposition of a substance , half - lives are g...
Text Solution
|
- At a certain temperature, the half life period for the catalytic decom...
Text Solution
|
- A first order reaction takes 10 minutes for 25% decomposition. Calcula...
Text Solution
|
- Half lives against initial pressure are given below. Calculate the ord...
Text Solution
|
- The half life period for catalytic decomposition of AB(3) at 50 mm is ...
Text Solution
|
- The half life period for catalytic decomposition of XY3 at 100 mm is f...
Text Solution
|
- The catalytic decomposition of hydrogen peroxide is a ...................
Text Solution
|
- For the catalytic decomposition of a substance , half - lives are give...
Text Solution
|
- 50 mm पर, AB(2), के उत्प्रेरकीय अपघटन के लिए अर्द्ध-आयु काल 4 घंटे प्र...
Text Solution
|