A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond dissociation energy depends upon the nature of the bond and n...
Text Solution
|
- The enthalpy change for the reaction, C(2)H(6)(g)rarr2C(g)+6H(g) is X ...
Text Solution
|
- If enthaopy of hydrogenation of C(6)H(6)(l) into C(6)H(12)(l) is -205k...
Text Solution
|
- If enthalpy of hydrogenation of C(6)H(6)(l) into C(6)H(12)(l) is -205 ...
Text Solution
|
- If enthalpy of dissociation of CH(4) and C(2)H(6) are 320 and 360 calo...
Text Solution
|
- Bond dissociation energy of CH(4) si 360 kJ/mol and C(2)H(6) has 620 k...
Text Solution
|
- Bond dissociation energy of CH(4) is 360 kJ/mol and C(2)H(6) is 620 kJ...
Text Solution
|
- The dissociation energy of CH(4) and C(2)H(6) are respectively 1508 an...
Text Solution
|
- The bond dissociation energy depends upon the nature of the bond and n...
Text Solution
|
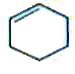 is ? Answer `Delta H_("vap") " of " C_(6)H_(6(l)), C_(6)H_(10(l)), C_(6)H_(12(l))` all are equal:
is ? Answer `Delta H_("vap") " of " C_(6)H_(6(l)), C_(6)H_(10(l)), C_(6)H_(12(l))` all are equal: