A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
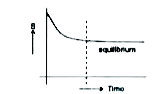
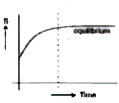
- Which correctly represents the entropy (s) of an isolated system durin...
Text Solution
|
- Assertion: In an isolated system the entropy increases. Reason: The pr...
Text Solution
|
- Assertion :- The entropy of isolated system increases for spontancous ...
Text Solution
|
- Which correctly represents the entropy (s) of an isolated system duri...
Text Solution
|
- For an isolated system, the entropy :
Text Solution
|
- Assertion :- In an isolated system the entropy increases due to sponta...
Text Solution
|
- Assertion : In an isolated system the entropy increases. Reason : Th...
Text Solution
|
- Assertion : In an isolated system the entropy increases. Reason : Th...
Text Solution
|
- Explain change in entropy of a system during a reversible process Delt...
Text Solution
|