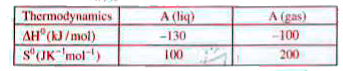A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|
- A porcess is nonspontaneous at evey temperature if (i) Delta H gt 0,...
Text Solution
|
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- For a given reaction , Delta H = 3.5 kJ mol^(-1) and Delta S = 83.6 J ...
Text Solution
|
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- For a given reaction, Delta H = 35.5 kJ mol^(-1) and Delta S = 83.6 JK...
Text Solution
|
- If Delta H and Delta U are the changes in the enthalpy and internal en...
Text Solution
|
- In which of the following cases,the reaction is spontaneous at all tem...
Text Solution
|
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|