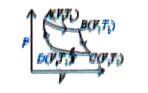A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- For this process (overall change) which is correct
Text Solution
|
- The correct equation that would represent the overall process of photo...
Text Solution
|
- For adiabatic process which is correct -
Text Solution
|
- संघनन की प्रक्रिया में निम्नलिखित में कौन परिवर्तन होता है?
Text Solution
|
- Distinguish the thermodynamic process depending upon heat absorbed or ...
Text Solution
|
- Draw the overall process of respiration in corresponding places.
Text Solution
|
- For adiabatic process, which is correct-
Text Solution
|
- किस प्रक्रम में एन्ट्रॉपी परिवर्तन नहीं होता है
Text Solution
|
- For this process (overall change) which is correct
Text Solution
|