Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
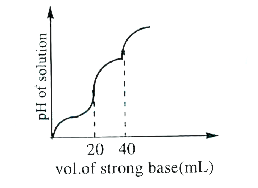
- 10 mLof H2A (weak diprotic acid) solutions is titrated against 0.1M Na...
Text Solution
|
- The correct relationship between the pH of isolomar solution of Na(2)O...
Text Solution
|
- The correct relationship between the pH of isomolar solutions of sodiu...
Text Solution
|
- What is the correct relationship between the pH of isomolar solutions ...
Text Solution
|
- During the titration of a weak diprotic acid (H(2)A) against a strong ...
Text Solution
|
- 10 mL of H(2)A (weak diprtic acid) solutio is titrated against 0.1M Na...
Text Solution
|
- What is the correct relationship between the pHs of isomolar solutions...
Text Solution
|
- Which of the following statemets are correct regarding : H(2)P-PH-PH...
Text Solution
|
- सोडियम ऑक्साइड (pH(1)), सोडियम सल्फाइड (pH(2)), सोडियम सेलेनाइड (pH(...
Text Solution
|