A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
UNITS AND MEASUREMENT
AAKASH SERIES|Exercise EXERCISE - II|61 VideosUNITS AND MEASUREMENT
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 VideosUNITS AND MEASUREMENT
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 VideosSEMICONDUCTOR DEVICES
AAKASH SERIES|Exercise EXERCISE - III|3 VideosUNITS AND MEASUREMENTS
AAKASH SERIES|Exercise EXERCISE -3|66 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-UNITS AND MEASUREMENT-EXERCISE - I
- The fundamental physical quantities that have same dimensions in the d...
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
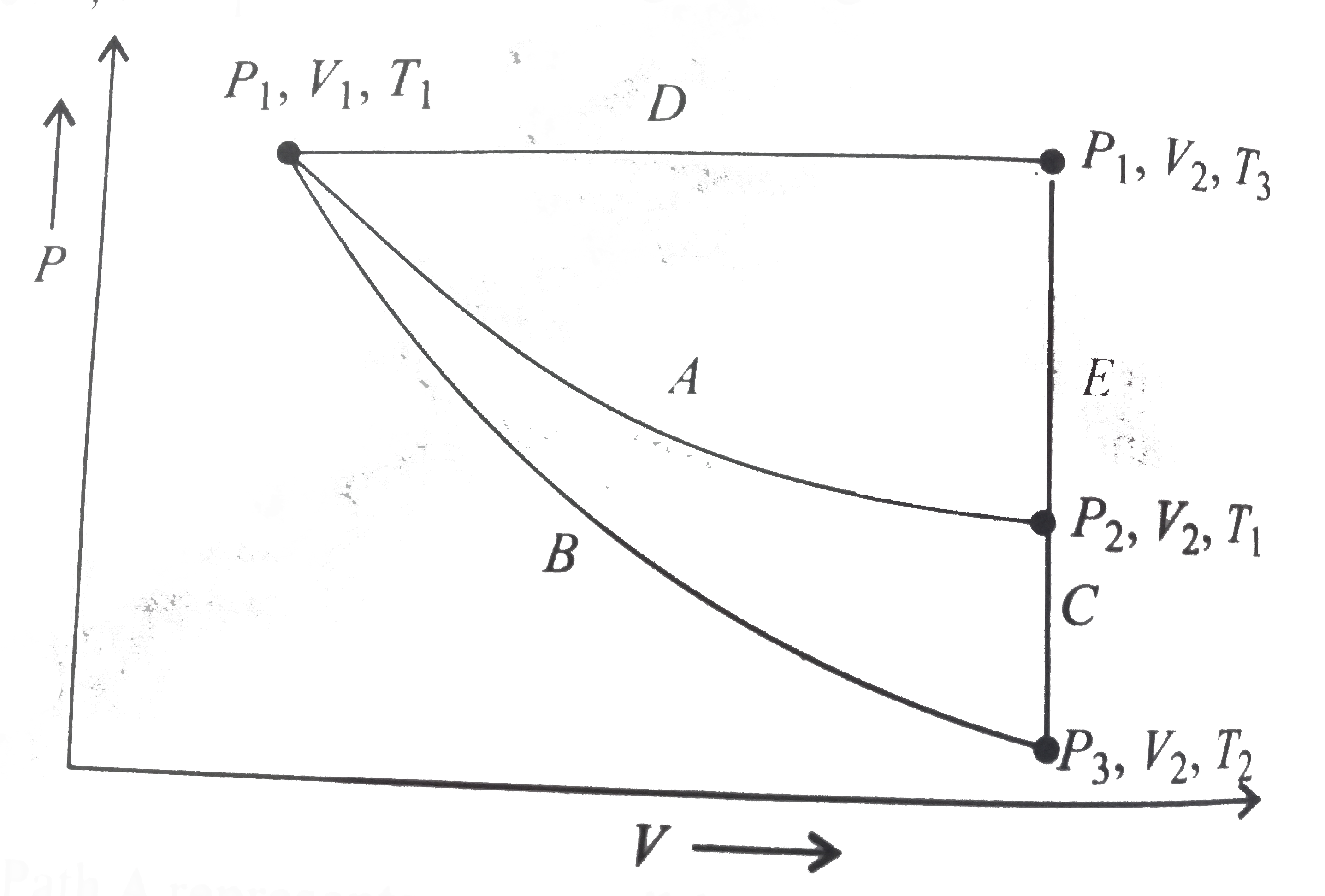
- For an ideal gas, an illustration of three different paths A,(B+C) and...
Text Solution
|
- The dimensional formula for angular momentum is
Text Solution
|
- The physical quantity that has no dimensions is
Text Solution
|
- The energy density and pressure have
Text Solution
|
- The dimensional formula ML^2T^(-2) represents
Text Solution
|
- The dimensional formula of torque is
Text Solution
|
- What is the dimensional formula of angular velocity?
Text Solution
|
- What is meant by faraday 's constant?
Text Solution
|
- Which of the following is the most precise instrument for measuring le...
Text Solution
|
- The value of g at depth h is two third the value that on the earth's ...
Text Solution
|
- The errors due to imperfect design or calibration of the measuring ins...
Text Solution
|
- For example, if you , by habit, always hold your head a bit too far to...
Text Solution
|
- By improving experimental techniques, selecting better instruments and...
Text Solution
|
- Unpredicatable fluctuations in temperature, voltage supply, mechanical...
Text Solution
|
- In a measurement, a choice of change of different units
Text Solution
|
- Zero error in an instrument introduces
Text Solution
|
- Which of the following is systematic error
Text Solution
|
- (A) : Increasing the number of observations minimizes random errors. ...
Text Solution
|