A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
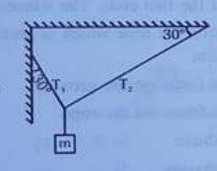
- Calculate T(1) & T(2).
Text Solution
|
- If the normals at points t1a n dt2 meet on the parabola, then t1t2=1 ...
Text Solution
|
- Calculate a, T(1), T(2), T(1)' & T(2)'.
Text Solution
|
- Calculate T(1) & T(2).
Text Solution
|
- Find the matrices of transformation T(1)T(2) and T(2)T(1) when T(1) is...
Text Solution
|
- If the tangents at t(1) and t(2) to a parabola y^2=4ax are perpendicul...
Text Solution
|
- The area of triangle formed by the points points and t(1),t(2) and t(3...
Text Solution
|
- Suppose (x+a)^(n)=T(0)+T(1)+T(2)+…+T(n) Then (T(0)-T(2)+T(4)-…)^(2...
Text Solution
|
- यदि (x+a)^(n) के विस्तार में, T(0),T(1),T(2),…. पद प्राप्त होते है, तो...
Text Solution
|