A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-TRANSPORT IN PLANTS -EXERCISE II
- Perianth is represented by
Text Solution
|
- With reference to magnetic dipole, match the tems of Column I with the...
Text Solution
|
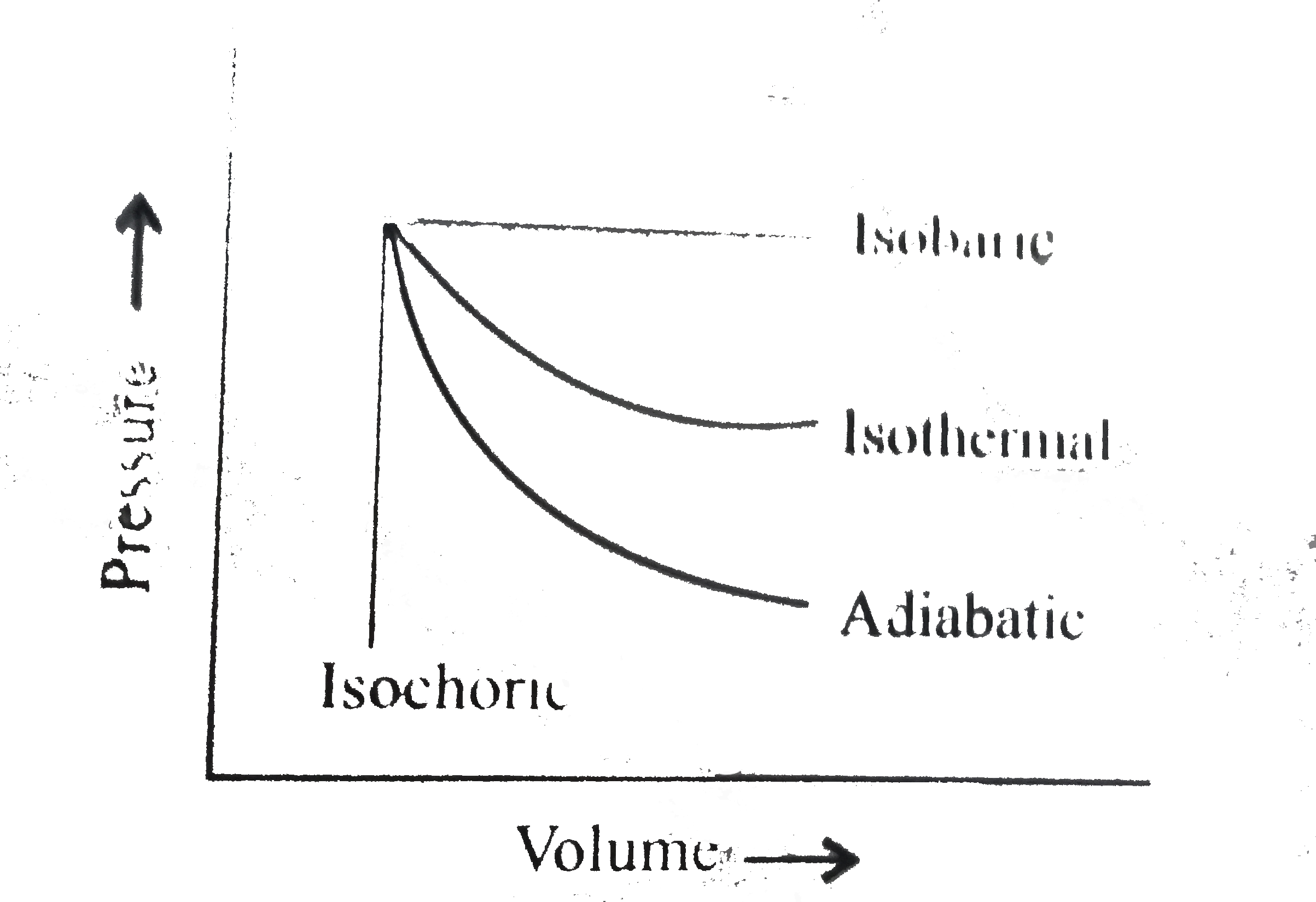
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- Perianth is represented by
Text Solution
|
- Who proposed relay pump theory of the ascent of sap ?
Text Solution
|
- The pressure developed in tracheary elements of xylem due to metabolic...
Text Solution
|
- The cut end of herbs stem just above the ground cause loss of water.Th...
Text Solution
|
- An innovative professor who wants to give a live demonstration of a ph...
Text Solution
|
- The cohesive force of water is due to :
Text Solution
|
- A higher plant cell covered with cutin and suberin is placed in water....
Text Solution
|
- Conduction of sap in plants occurs through:
Text Solution
|
- The blockage of one or two tracheary elements in a tree may cause
Text Solution
|
- This plant is ideal to demonstrate ascent of sap
Text Solution
|
- The term ‘tensile strength’ represents that there is
Text Solution
|
- In a branch cut from a rapidly transpiring plant, water snaps away fro...
Text Solution
|
- When an oak tree is kept in a poisonous solution, that rises to the to...
Text Solution
|
- Who among the following scientists first gave the starch sugar interco...
Text Solution
|
- Chemical reactions are invariably associated with the transfter of ene...
Text Solution
|
- A chemical widely used as antitranspirant
Text Solution
|
- Which of the following theory gives the latest explanation for the ope...
Text Solution
|