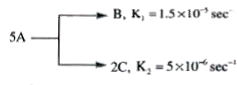A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A follows parallel path first order reaction to give B and C as given ...
Text Solution
|
- A follow parallel path of first-order reactions giving B and C as If t...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measur...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measu...
Text Solution
|
- In a first order reaction A to B, if k is rate constant and initial co...
Text Solution
|
- A तथा B के मध्य अभिक्रिया के लिये, अभिक्रिया की प्रारंभिक दर को A तथा ...
Text Solution
|
- Which of the following relation is correct for a first order reaction?...
Text Solution
|
- The initial rates for gaseous reaction A+3BtoAB(3) are given below [A]...
Text Solution
|
- A follows parallel path first order reaction to give B and C as given ...
Text Solution
|