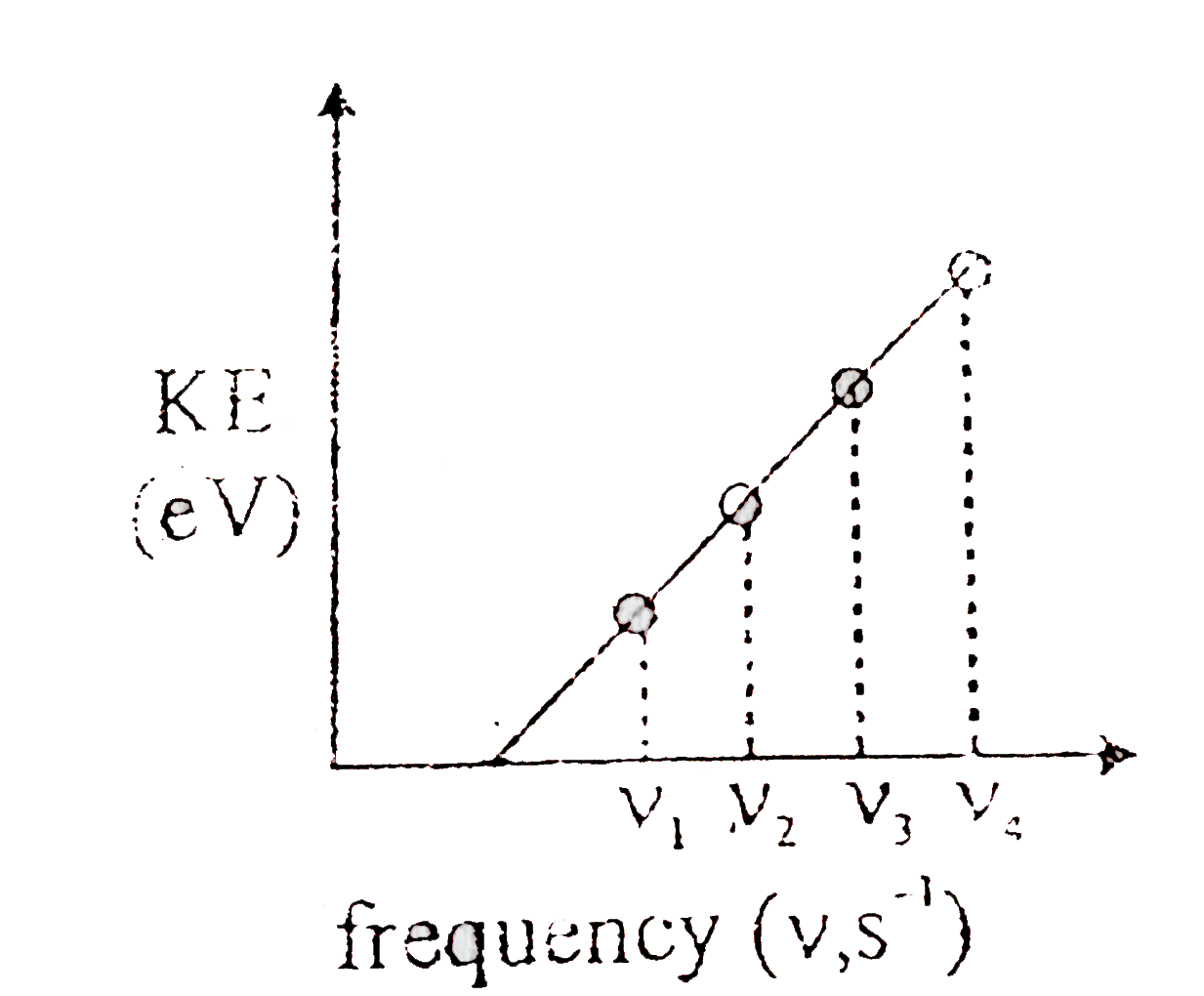
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a photoelectric experiment, kinetic energy of photoelectrons was pl...
Text Solution
|
- The maximum kinetic energy of the emitted photoelectrons against frequ...
Text Solution
|
- If the frequency of light in a photoelectric experiment is double then...
Text Solution
|
- In a photoelectric experiment, kinetic energy of photoelectrons was pl...
Text Solution
|
- For the photoelectric effect, the maximum kinetic energy E(k) of the e...
Text Solution
|
- Figure represents a graph of kinetic energy (K) of photoelectrons (in ...
Text Solution
|
- Kinetic energy of surface photoelectrons is x when frequency of incide...
Text Solution
|
- The photoelectric threshold frequency of a metal is v. When light of f...
Text Solution
|
- For photoelectric effect, the graph of the maximum kinetic energy K of...
Text Solution
|