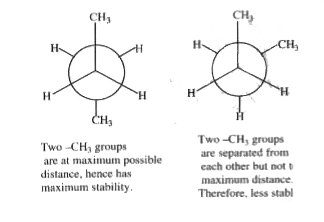
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Draw Newman projection of the less stable staggered from of butane. ...
Text Solution
|
- Relative Stability Of Staggered And Eclipsed Conformations Of Ethane
Text Solution
|
- Draw Newman projection of the less stable staggered from of butane. b....
Text Solution
|
- In the Newman projection formula of the lost stable staggered form of ...
Text Solution
|
- (a) Draw Newman's projection for the stable staggered form of butane. ...
Text Solution
|
- Draw Newman and sawhorse projections for the eclipsed and staggered co...
Text Solution
|
- Draw the staggered and eclipsed conformers of n-butane .
Text Solution
|
- Draw the eclipsed and staggered conformations of ethane using Newman p...
Text Solution
|
- Draw the eclipsed and staggered conformations of ethane and comment on...
Text Solution
|