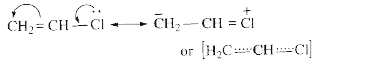Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Why the chlorine atom in vinyl chloride is nonreactive?
Text Solution
|
- Chloride ion and chlorine atom have
Text Solution
|
- Asertion (A) : Vinyl chloride can be differentied from ethyl chloride...
Text Solution
|
- What is the number of lone pairs in chlorine atom in vinyl chloride?
Text Solution
|
- क्लोरोबेन्जीन का क्लोरीन परमाणु एथिल क्लोराइड के क्लोराइड परमाणु की तु...
Text Solution
|
- क्लोरोबैंजीन का क्लोरीन परमाणु ऐथिल क्लोराइड के क्लोरीन परमाणु की तुलन...
Text Solution
|
- बेन्जिलिक क्लोराइड तथा वाइनिलिक क्लोराइड के संरचना सूत्र लिखिए। इन यौ...
Text Solution
|
- Chlorine in vinyl chloride is less reactive because
Text Solution
|
- Why is vinyl chloride less reactive than ethyl chloride ?
Text Solution
|