A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
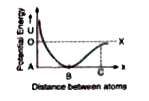
- The potential energy (U) between two atoms in a diatomic molecule as a...
Text Solution
|
- The potential energy U between two molecules as a function of the dist...
Text Solution
|
- The potential energy U between two molecules as a function of the dist...
Text Solution
|
- The potential energy between two atoms in a molecule is given by U(x)=...
Text Solution
|
- The potential energy U between two molecules as a function of the dist...
Text Solution
|
- The potential energy function for the force between two atoms in a dia...
Text Solution
|
- The potential energy function for the force between two atoms in a dia...
Text Solution
|
- एक द्विपरमानुक अणु में दो परमाणुओं के बीच लग रहे बल के लिए स्थितिज ऊ...
Text Solution
|
- The potential energy (U) between two atoms in a diatomic molecule as a...
Text Solution
|